With antibiotic-resistant bacteria on the rise, scientists are urgently trying to find drugs that will work against persistent infections. But coming up with new ones does not have to be the only strategy.
A recent study published in Proceedings of the National Academy of Sciences found that they can repurpose bithionol — a drug formerly used to treat parasitic infections in horses — to kill antibiotic-resistant bacteria, including MRSA, a common hospital-acquired infection.
The results not only suggest a promising treatment for this infection but hint at new ways scientists could tackle the problem of antibiotic resistance, by exploring new uses for old drugs and also using them in combination with traditional antibiotics.
“There’s a whole lot of effort these days to try and repurpose compounds to address the antibiotic resistance challenge,” says Gerry Wright, a professor in the department of biochemistry and biomedical sciences at McMaster University in Canada who studies how bacteria become resistant to antibiotics.
“It’s nice to see a result like this because it does tell us that there’s lots of interesting molecules out there, even molecules that used to be used for something else,” says Wright, who was not involved in the study.
The work was a collaborative effort among researchers at Brown, Emory and Harvard universities. Their idea was to find an existing drug that could target the bacteria’s membrane, a part of the bacteria that most antibiotics don’t attack.
The membrane “is the only target that you can go after that the bacteria can’t really avoid,” says Dr. Eleftherios Mylonakis, professor of infectious diseases at Brown University and corresponding author of the study.
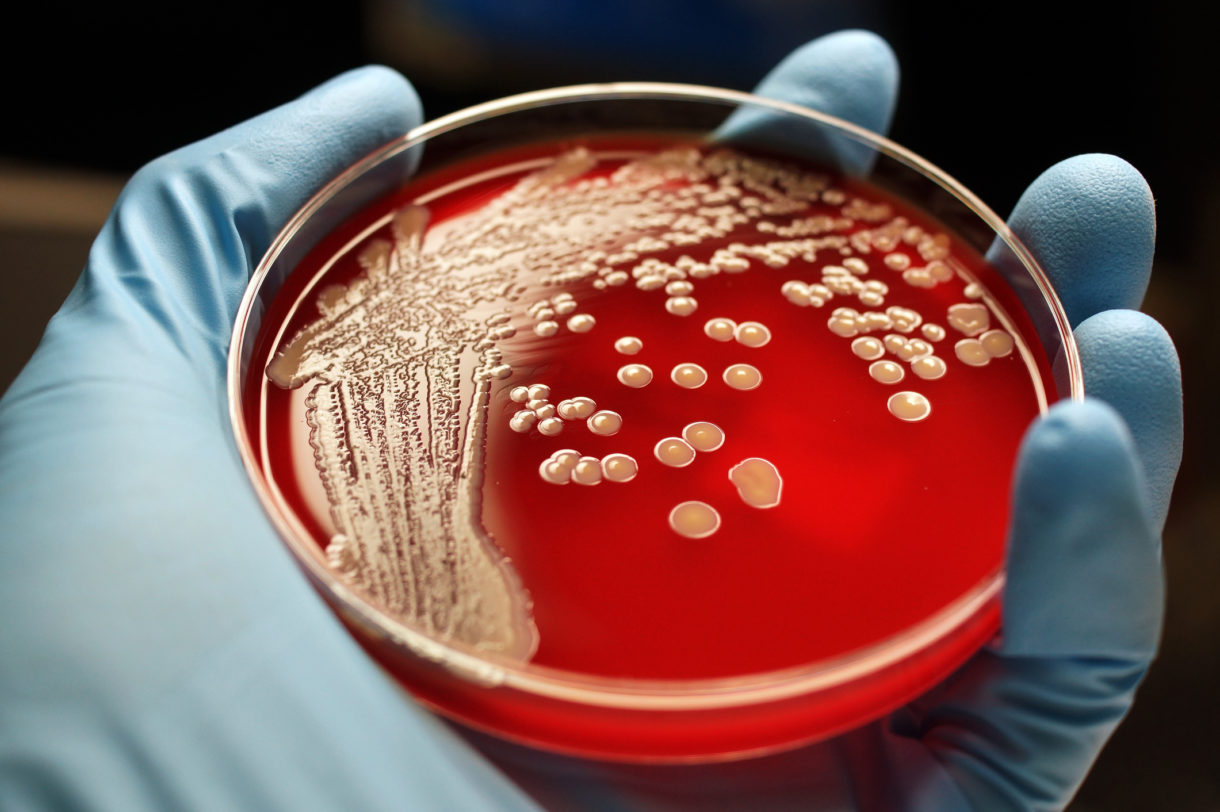
MRSA, or methicillin-resistant Staphylococcus aureus, is the bug responsible for some cases of pneumonia, as well as skin and blood infections. The bacteria are especially dangerous for older or sick patients who don’t have strong immune systems.
MRSA infections contain bacteria known as persisters, which protect themselves against antibiotics by becoming “couch potatoes,” says William Wuest, associate professor of chemistry at Emory University and one of the authors of the study. They go into a low-energy, dormant state, and this allows them to escape the attack of common antibiotics, which target the ability of the bacteria to grow and reproduce.
A membrane that controls what comes in and out surrounds the inside of all bacteria. The membrane is always there, even if the bacteria are not actively growing. This makes the bacterial membrane an attractive point from which to attack persisters.
The scientists started by looking for existing drugs that could penetrate the membrane of MRSA persisters.
They performed a screen of more than 80,000 known drugs. They infected worms known as C. elegans with MRSA and then tried the drugs to see which would kill the MRSA bacteria, but not the worms. They found 185 drugs that passed this screen, but only a few of them, including bithionol, could penetrate the membrane of persister bacteria.
The scientists then showed that bithionol could kill MRSA persisters in a lab dish within 24 hours, while conventional antibiotics like daptomycin and linezolid could not.
While it was known that bithionol had some antibacterial activity, the results from this study indicate that it “is actually much more potent than what had been previously reported,” says Meghan Blackledge, assistant professor of chemistry at High Point University, who researches treatments for MRSA. Blackledge was not involved in this study.
But the researchers found a potential problem with bithionol — it’s destructive. When used in high doses, it makes the bacteria burst open and die. For bithionol to be a safe antibiotic treatment in humans, the scientists had to show that it could penetrate only the membranes of bacteria, not those of human cells.
Unlike the membranes of bacteria, the membranes of human cells and those of other mammals contain cholesterol, which makes for a more densely packed and difficult-to-penetrate membrane. Using computer simulations, the scientists showed that bithionol could penetrate only the membranes of bacteria, not those of mammalian cells.
Another problem with antibiotics that kill bacteria by bursting them open is that they might generate resistance. This is because bacteria adapt to being attacked and can change in ways that reduce the effectiveness of antibiotics.
The researchers designed an experiment to see whether the drug could work without bursting the bacteria’s membranes. They tried the drug in mice that had an established MRSA infection in their thighs. They used a lower dose of bithionol that couldn’t kill the MRSA persisters on its own and combined it with the traditional antibiotic gentamicin. The combination eradicated 90% of the MRSA thigh infection, while other common antibiotics alone did not.
The lower dose of bithionol penetrated the membrane of MRSA persisters, but instead of breaking the membrane open, it loosened it up to allow gentamicin to kill the bacteria from the inside.
An advantage of bithionol is that it can be used at a concentration at which it’s not able to kill MRSA on its own and that it can be combined with traditional antibiotics that can finish the job. For example, gentamicin isn’t normally used to treat infections in humans because the dose at which it works might cause kidney damage. But by combining it with bithionol the researchers were able to use gentamicin at lower concentrations.
“I really think that the future for overcoming antibiotic resistance is these combination therapies where we can use lower doses of the more toxic antibiotics,” says Wuest.
Wright says the potential for bacteria to become resistant to bithionol still exists. “In my view, there’s no such thing as a compound that bacteria cannot get resistance to,” says Wright. “It’s not obvious to me how it’ll happen, but I’m quite sure that at some point it will happen.”
Wuest agrees. “I would hesitate to say that [bithionol] would never be able to have resistance generated against it,” he says. “But the mechanism by which it works … makes me more optimistic.”
Luisa Torres is an AAAS mass media fellow on NPR’s science desk. She’s on Twitter: @luisatorresduq
9(MDEwNzczMDA2MDEzNTg3ODA1MTAzZjYxNg004))